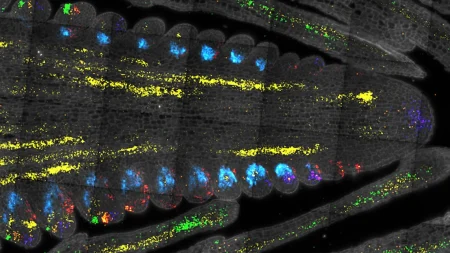
In a surprising turn of events for a team of researchers from Memorial Sloan Kettering Cancer Center (MSK) and their collaborators at the Icahn School of Medicine at Mount Sinai, an unexpected discovery has led to a potential improvement in therapies that use small RNAs to silence disease-causing genes, including those related to cancer. While conducting an experiment on the protein ALAS1’s role in making small regulatory RNAs known as microRNAs, the researchers found that removing the protein from cells actually increased levels of microRNAs, contrary to their expectations. This led to the revelation of a previously unrecognized role for ALAS1 beyond its known function in heme production. Heme plays a crucial role in various biological processes, including oxygen transport, energy production, and microRNA synthesis.
MicroRNAs and small interfering RNAs (siRNAs) are short snippets of RNA that bind to specific messenger RNAs and repress them, effectively silencing genes. Scientists have successfully utilized this mechanism to create RNA-based drugs that target disease-causing genes. The first siRNA drug, patisiran, was approved by the FDA in 2018 to treat hereditary transthyretin amyloidosis, with several others in development or clinical trials. The discovery made by the MSK researchers has the potential to enhance the efficacy of siRNA drugs by targeting ALAS1, which acts as a brake on the production of microRNAs. Removing this brake could improve the ability of siRNA drugs to silence target genes, especially those associated with diseases like cancer.
The research team, led by Dr. Seungjae Lee, partnered with experts from Mount Sinai specializing in heme regulation and ALAS genes to delve further into their findings. By extending their experiments to custom animal models, the researchers validated their discovery that removing ALAS led to a global increase in microRNAs in mice. This knowledge could be harnessed to enhance the gene-silencing activity of siRNA drugs against various disease-causing genes, potentially expanding their application beyond liver cells. Combining an siRNA against ALAS1 with other siRNA drugs may improve their efficacy, reduce side effects, and broaden their reach to treat a wider range of diseases.
The recent recognition of Harvard geneticist Gary Ruvkun and Victor Ambros with the Nobel Prize for their discovery of microRNA underscores the importance of curiosity-driven research in advancing scientific knowledge and medical breakthroughs. Dr. Lai, who credits Dr. Ruvkun for his career in developmental biology and small RNAs, emphasizes the value of fundamental research in driving major advancements in understanding gene regulation and developing innovative therapies. By supporting foundational research in model organisms and cellular processes, society can foster the discovery of novel treatments and solutions to complex diseases like cancer, highlighting the significance of ongoing investment in scientific research across various fields.
Funding for the research was provided by grants from the National Institutes of Health and other sources, supporting the team’s exploration of new pathways to enhance the efficacy of RNAi therapy through targeting ALAS1 and ALAS2. The researchers have also filed a patent application based on their methods for improving RNAi therapy by focusing on these enzymes. Drs. Yasuda and Desnick, who are co-inventors of a patent for RNAi therapy for acute hepatic porphyrias, disclose pharmaceutical consulting work related to their findings. This collaborative research effort exemplifies the impact of unexpected discoveries in driving progress in therapeutic development and underscores the importance of continued support for foundational research to address complex medical challenges.