A hallmark of Alzheimer’s disease and other neurodegenerative diseases is the clumping together of insoluble protein aggregates in the brain, which also occurs during normal aging. Previous approaches to treating Alzheimer’s disease have focused on specific insoluble proteins, but a recent study by Buck researchers in worms revealed a complex relationship between insoluble proteins, aging, and neurodegenerative diseases. The research identified a potential intervention to reverse the toxic effects of protein aggregates by boosting mitochondrial health, offering new strategies for preventing and treating age-related diseases.
The study, published in the journal GeroScience, demonstrates how targeting insoluble proteins could play a key role in addressing a variety of age-related diseases. By maintaining healthy mitochondria, it is possible to combat protein clumping associated with aging and Alzheimer’s disease. The findings support the geroscience hypothesis, which suggests a common pathway to Alzheimer’s disease and aging. According to Gordon Lithgow, Vice President of Academic Affairs at Buck Institute, the factors that contribute to Alzheimer’s disease may begin early in life, highlighting the importance of understanding the connections between protein insolubility, aging, and disease.
Most research on Alzheimer’s disease has focused on amyloid beta and tau proteins, but there are thousands of other proteins involved in insoluble aggregations whose role in the disease is unknown. The study revealed that amyloid beta causes a significant amount of insolubility in proteins, even in young animals, suggesting a vicious cycle where aging and amyloid beta contribute to protein aggregation. The core insoluble proteome identified in the study contains proteins linked to various neurodegenerative diseases, shedding light on the broader impact of protein insolubility on aging and age-related diseases.
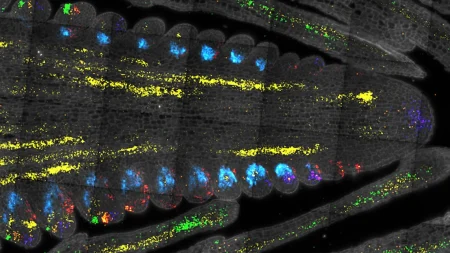
The researchers used a strain of Caenorhabditis elegans engineered to produce human amyloid protein to investigate the connection between protein aggregates in Alzheimer’s disease and normal aging. They found that amyloid beta drives insolubility in other proteins and accelerates the aggregation of amyloid when mixed with insoluble proteins from aged animals. The study also demonstrated that boosting mitochondrial health using a natural compound called Urolithin A can mitigate the toxic effects of amyloid beta, offering a potential therapeutic approach for Alzheimer’s disease and other age-related conditions.
The importance of mitochondria in maintaining overall health and addressing protein insolubility was a key finding from the study. The authors emphasized the critical role of mitochondria in the aging process and in amyloid beta-related toxicity, highlighting the need to support mitochondrial health through exercise and a healthy diet. By understanding the links between insoluble proteins, aging, and disease, researchers can develop new strategies for preventing and treating age-related conditions, offering hope for more effective therapies in the future.