Biomedical engineers at Duke University have created a silk-based ultrathin membrane that can be utilized in organ-on-a-chip models to better mimic the natural environment of cells and tissues in the body. By using this membrane, researchers have been able to grow tissues that replicate the functionality of both healthy and diseased kidneys. This new membrane allows cells to grow closer together, enabling researchers to better control the growth and function of key cells and tissues within any organ, leading to more accurate disease modeling and therapeutic testing.
Organ-on-a-chip (OOC) systems have become essential tools for studying human biology by creating dynamic models of tissue structures, studying organ functions, and modeling diseases. These systems are tailored to facilitate cell growth and differentiation in a manner that closely resembles the target organ. Researchers can even populate these tools with human stem cells to generate patient-specific organ models for preclinical studies. However, challenges have arisen in the design of these chips, particularly with the materials used to create the membranes that support the specialized cells. The typical polymer membranes are thick and do not mimic the extracellular membranes found in human organs, hindering communication between cells.
By exploring the use of silk fibroin, a protein produced by silkworms, researchers were able to create an ultrathin and porous membrane that closely resembles the structure of the extracellular matrix in human organs. This membrane allows for cells to be handled in a manner similar to biopsy samples or living tissues, enabling the formation of structures that closely resemble human tissues. The use of silk fibroin has significantly reduced the membrane thickness, bringing it closer to what is seen in living organisms, and improving the communication between cells and the overall growth of tissues within the organ-on-a-chip models.
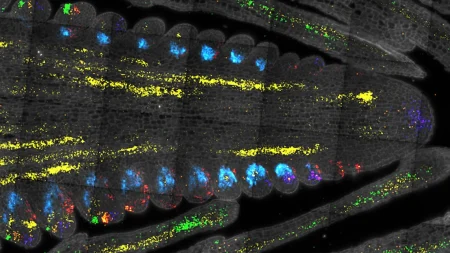
In a study involving kidney chip models, the silk fibroin membrane was applied, and human induced pluripotent stem cell derivatives were added. The cells were able to communicate across the ultrathin membrane, facilitating the differentiation into glomerular cells, podocytes, and vascular endothelial cells. The platform also triggered the development of endothelial fenestrations within the growing tissue, enabling the passage of fluid between cellular layers. By the end of the testing, various kidney cell types had assembled into a glomerular capillary wall and were efficient in filtering molecules by size, showcasing the potential of the new microfluidic chip system in advancing the understanding of human organ development, disease progression, and therapeutic development.
Further optimization of the model aims to enhance the understanding of kidney disease mechanisms, as effective models for the disease are currently lacking. The technology could help discover new biomarkers for kidney disease and aid in the screening of drug candidates for multiple kidney disease models. The potential applications of this platform extend to all organ-on-a-chip models, enabling the improvement of models for various organs and disease states such as the brain, liver, and lungs. The versatility of this technology presents exciting possibilities for advancing research in organ modeling and disease understanding.
This research was supported by various grants and awards, including a Whitehead Scholarship in Biomedical Research, Chair’s Research Award from the Department of Medicine at Duke University, MEDx Pilot Grant on Biomechanics, Duke Incubation Fund, and an NIH Director’s New Innovator Grant. The implications of this work not only have the potential to advance the field of organ-on-a-chip modeling but also to significantly impact the study of human organ development, disease progression, and therapeutic development.