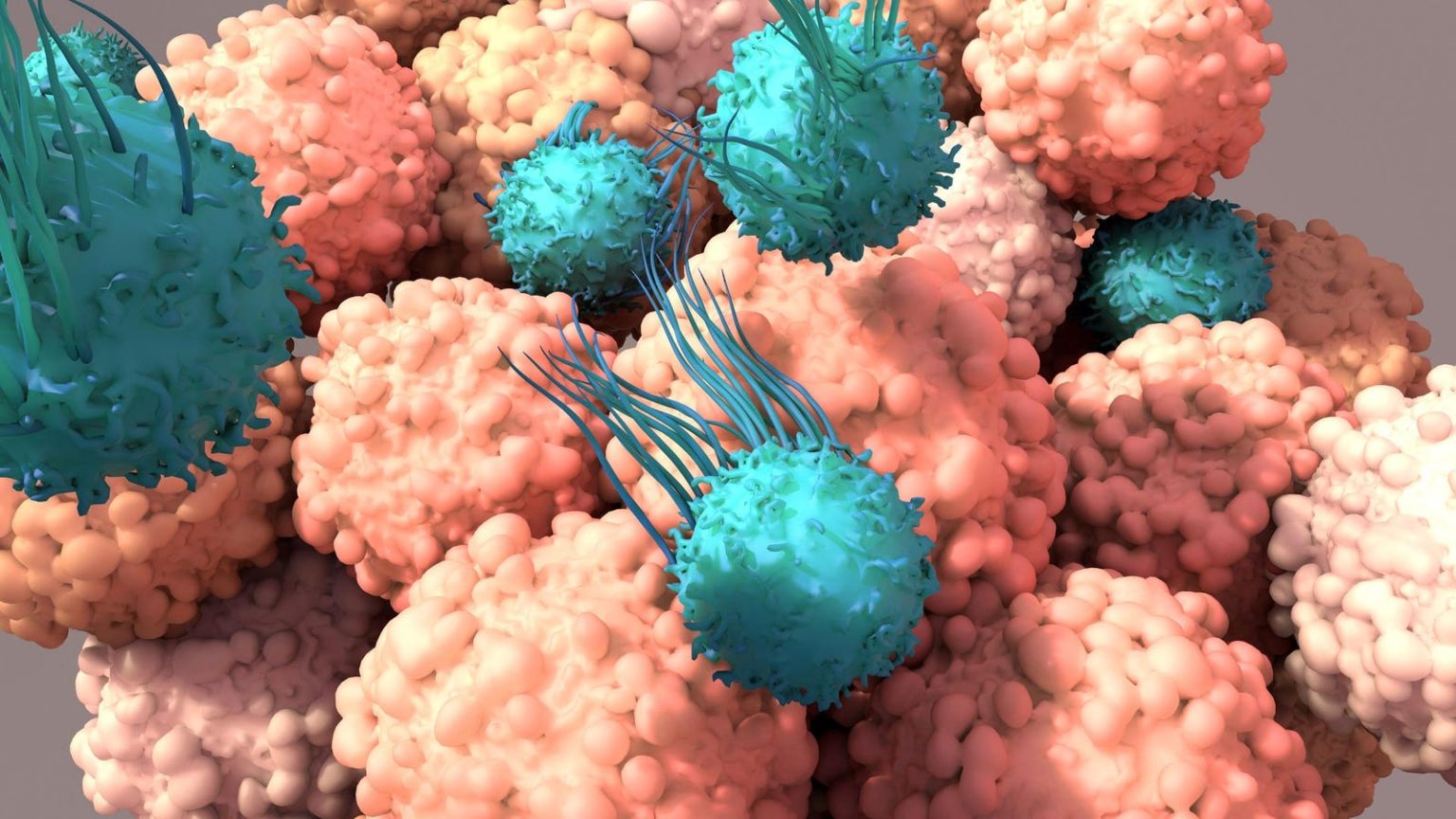
In 2011, a groundbreaking new generation of anticancer drugs called checkpoint inhibitors was introduced with ipilimumab, also known as Yervoy. This drug was designed to prevent cancer cells from evading immune cell attacks, leading to a new era of cancer treatment. Since then, several other checkpoint inhibitors have been developed, expanding the range of cancers that can be treated with these medications.
Ipilimumab works by blocking a specific protein on immune T cells known as immune checkpoints. By doing so, it encourages these white blood T cells to fight against tumors. These immune checkpoint proteins serve as a natural “braking system” for the immune system, preventing overactivation that could harm healthy tissues. Cancer cells exploit this mechanism by binding to checkpoint proteins, deactivating immune responses against tumors.
The unique aspect of ipilimumab is its ability to target a specific protein called CTLA-4, differentiating it from other checkpoint inhibitors. While ipilimumab can be used as a standalone treatment, it is often combined with other checkpoint inhibitors or traditional cancer therapies. It has been approved for the treatment of aggressive melanoma and has shown effectiveness in a variety of difficult-to-treat cancers that cannot be fully removed through surgery alone or have metastasized.
Like all medications, ipilimumab can cause side effects, most commonly related to stimulating the immune system. These adverse reactions can range from fatigue and skin rash to more severe conditions like colon inflammation. When combined with nivolumab, another checkpoint inhibitor, additional side effects such as headaches and abdominal pain may occur. Regular monitoring and prompt management of these immune-related adverse events are essential.
Ongoing clinical trials are exploring new applications for ipilimumab, often in combination with other treatments, to optimize dosing, reduce side effects, and improve efficacy. While the drug has shown limited effectiveness as a single agent for some cancers, it has demonstrated promising results in combination regimens for various solid tumors. Research is being conducted in cancers such as small cell lung cancer, prostate cancer, and bladder cancer to further expand the therapeutic potential of ipilimumab.
In conclusion, ipilimumab represents a significant advancement in cancer treatment and has paved the way for other checkpoint inhibitors. This medication has shown the potential to unleash the body’s natural defenses against cancer and can be used in combination with other therapies to enhance antitumor responses. Continued research and clinical trials will continue to explore the capabilities of ipilimumab and expand its therapeutic reach in the fight against cancer.